Efficacy & safety
Safe and effective for a
broad range of cIAI patients
Trial Design
Efficacy demonstrated in
2 rigorous phase 3 trials 1,2
Evaluating the efficacy and safety of XERAVA versus either ertapenem or meropenem in patients with cIAI1-3
Study Design:
IGNITE1 and IGNITE4
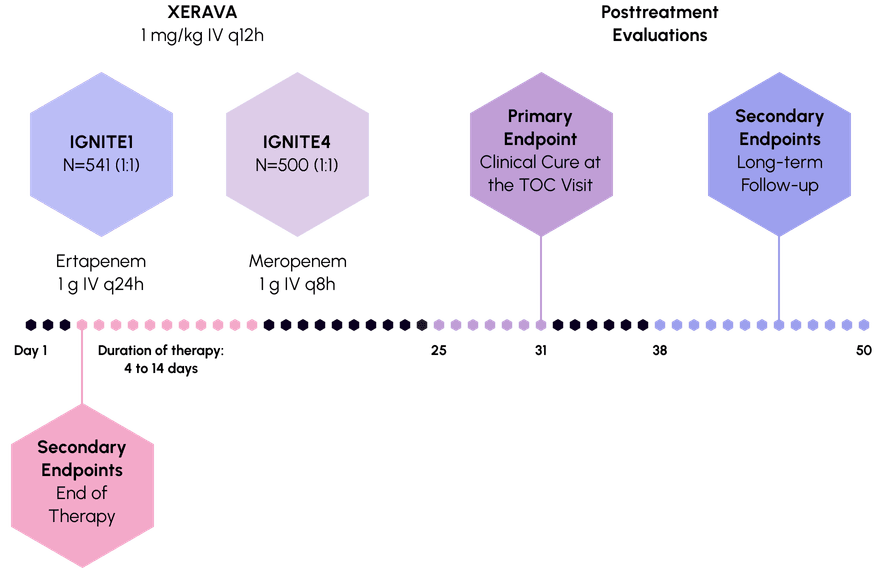
Primary endpoint: Clinical cure was defined as complete resolution or significant improvement of signs or symptoms of the index infection at the Test of Cure (TOC) visit, which occured 25 to 31 days after randomization.
IGNITE1: Phase 3, randomized, double-blind, double-dummy, multicenter, prospective study in subjects with cIAI to demonstrate the non-inferiority of XERAVA compared with ertapenem.
IGNITE4: Phase 3, randomized, double-blind, double-dummy, multicenter, prospective study in subjects with cIAI to demonstrate the non-inferiority of XERAVA compared with meropenem.

for a broad range of patients ciai, covered
Clinical Response
Delivered robust efficacy
as monotherapy 1,2,4
Clinical responses in micro-itt population at the TOC Visit
Slide chart to view more
CI=Confidence Interval; ITT=intention to treat.
Concurrent bacteremia was present in 32 patients in the XERAVA group and 31 patients in the comparator group in the pooled micro-ITT population in IGNITE1 and IGNITE4. In a post-hoc analysis, the rates of clinical response were4: XERAVA: 88% Comparators: 77%
Clinical Cure
Achieved high clinical cure rates
Clinical cure rate in micro-ITT population at the TOC visit
Slide table to view more
| Pathogen | XERAVAa N=415 % (n/N1) | Comparatorsb N=431 % (n/N1) |
|---|---|---|
| C. freundii | 86% (19/22) | 80% (8/10) |
| E. cloacae complex | 81% (17/21) | 96% (23/24) |
| E. coli | 87% (220/253) | 89% (237/266) |
| K. oxytoca | 93% (14/15) | 84% (16/19) |
| K. pneumoniae | 95% (37/39) | 84% (42/50) |
| E. faecalis | 83% (45/54) | 87% (47/54) |
| E. faecium | 84% (38/45) | 91% (48/53) |
| S. aureus | 100% (24/24) | 86% (12/14) |
| S. anginosus groupc | 86% (79/92) | 85% (50/59) |
| Anaerobesd | 87% (186/215) | 91% (194/214) |
Pooled data from IGNITE1 and IGNITE4
aXERAVA dose equals 1 mg/kg every 12 hours IV.
bComparators include ertapenem 1 g every 24 hours IV and meropenem 1 g every 8 hours IV.
cIncludes Streptococcus anginosus, Streptococcus constellatus, and Steptococcus intermedius.
dIncludes Bacteroides caccae, Bacteriodes fragilis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus, Clostridium perfringens, and Parabacteroides distasonis.
Successful against resistant strains a,5,6
Clinical cure rate in micro-itt population at the toc visit
Slide chart to view more
aEnterobacteriaceae: n=314 for XERAVA and n=325 for pooled comparators.
bComparators include ertapenem and meropenem.
cMDR pathogens were defined as resistant to at least 1 member of 3 or more antibiotic classes.
CEPH-R=cephalosporin-resistant; MDR=multidrug-resistant.
Safety
Well tolerated
in 2 pivotal
clinical trials
Low rates of nausea
and vomiting
Rates of nausea and vomiting reported in patients with cIAI receiving XERAVA in controlled clinical studies
low rate of ae-related discontinuation
Rate of discontinuation reported in patients with cIAI receiving XERAVA in controlled clinical studies
The most commonly reported adverse reactions leading to discontinuation of XERAVA were related to gastrointestinal disorders.
The most commonly reported adverse reactions leading to discontinuation of XERAVA were related to gastrointestinal disorders.
aComparators include ertapenem and meropenem.
AE=adverse events.
Real-world Data
Impactful real-world results
Comparing XERAVA with other antibiotics in patients with cIAI7
Want to
know more?
Request to speak with a
XERAVA sales representative
or receive information.
Lead the
charge
Choose broad-spectrum
coverage for a broad range of
cIAI patients with XERAVA.8
References:
- Solomkin JS, Gardovskis J, Lawrence K, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis. 2019;69(6):921-929. doi: 10.1093/cid/ciy1029.
- Solomkin J, Evans D, Slepavicius A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg. 2017;152(3):224-232. doi: 10.1001/jamasurg.2016.4237.
- Heaney M, Mahoney MV, Gallagher JC. Eravacycline: the tetracyclines strike back. Ann Pharmacother. 2019 Nov;53(11):1124-1135. Doi: 10.1177/1060028019850173.
- Grant-Di Felice V, Efimova E, Izmailyan S, et al. Efficacy and tolerability of eravacycline in bacteremic patients with complicated intra-abdominal infection: a pooled analysis from the IGNITE1 and IGNITE4 studies. Surg Infect. 2021:22(5):556-561. doi: 10.1089/sur.2020.241.
- Ditch K, Newman J, Izmailyan S, et al. Microbiological efficacy of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including MDR isolates: a pooled analysis from IGNITE1 and IGNITE4, two phase 3 trials of complicated intra-abdominal infection. Poster presented at: ASM Microbe; June 7-11, 2018; Atlanta, GA. Poster P629.
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multi-drug resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. doi: 10.1111/j.1469-0691.2011.03570.x.
- Meng R, Guan X, Sun L, et al. The efficacy and safety of eravacycline compared with current clinically common antibiotics in the treatment of adults with complicated intra-abdominal infections: A Bayesian network meta-analysis. Front Med. 2022. 9:935343. doi: 10.3389/fmed/2022/935343
- Zhanel GG, Cheung D, Adam H, et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs. 2016;76(5):567-588. doi: 10.1007/s40265-016-0545-8.
Indications & Usage
Indications
XERAVA® (eravacycline) for injection is indicated for the treatment of complicated intra-abdominal infections (cIAI) caused by susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae, Klebsiella oxytoca, Enterococcus faecalis, Enterococcus faecium, Staphylococcus aureus, Streptococcus anginosus group, Clostridium perfringens, Bacteroides species, and Parabacteroides distasonis in patients 18 years or older.
Limitations of Use XERAVA is not indicated for the treatment of complicated urinary tract infections (cUTI).
Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of XERAVA and other antibacterial drugs, XERAVA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Important Safety Information
Contraindications:
XERAVA is contraindicated for use in patients with known hypersensitivity to eravacycline, tetracycline-class antibacterial drugs, or to any of the excipients. Life-threatening hypersensitivity (anaphylactic) reactions have been reported with XERAVA.
Warnings and Precautions:
- The use of XERAVA during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia.
- The use of XERAVA during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth.
- Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, and may range in severity from mild diarrhea to fatal colitis.
Adverse Reactions:
The most common adverse reactions observed in clinical trials (incidence ≥3%) were infusion site reactions (7.7%), nausea (6.5%), and vomiting (3.7%).
XERAVA is structurally similar to tetracycline-class antibacterial drugs and may have similar adverse reactions. Adverse reactions including photosensitivity, fixed drug eruption, pseudotumor cerebri, and anti-anabolic action which has led to increased BUN, azotemia, acidosis, hyperphosphatemia, pancreatitis, and abnormal liver function tests, have been reported for other tetracycline-class antibacterial drugs, and may occur with XERAVA. Discontinue XERAVA if any of these adverse reactions are suspected.
You are encouraged to report negative side effects of prescription drugs to the FDA. To report SUSPECTED ADVERSE REACTIONS, please contact:
Innoviva Specialty Therapeutics, Inc.™
1‑800‑651‑3861
U.S. Food and Drug Administration
1‑800‑FDA‑1088
Before administering, please see the Full Prescribing Information for XERAVA.